Incorrect Does HNO have a net dipole. VIDEO Finding the Central Atom of an Electron Dot Structure Lewis Structure Examples.
Eight electrons come from one double bond pair of B-Cl and two single bond pairs of B-Cl on the boron central atom of BCl3.

. Draw the Lewis structure of HNO N is the central atom. With the help of three single bonds it already shares six electrons. The core atom in the BCl3 Lewis structure is boron which is bonded to the three chlorine atoms by single bonds B-Cl.
Determine how many electrons must be added to central element. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. What this means is that we decide how the atoms are to be bonded.
Hydrogen is never the central atom. A Lewis diagram is a representation of the valence shell electrons in a molecule where each atom participating in the bond tries to acquire the octet structure. Draw the Lewis structure.
How to Draw a Lewis Dot Structure Step 1. A If there are more than one of the least electronegative atom your skeletal structure should have those two attached to one another EXCEPTION. Draw the lewis dot structure for each of the following polyatomic ions.
In drawing the Lewis structure the central atom is generally the. The order of electronegativity for the nonmetals is FONClBrISCH. June 21 2021 by Admin.
What is the formal charge on CL in the following Lewis structure So the correct answer is 3. Here is an Electronegativity Table. 2 Use The.
Determine the total number of electrons available for bonding. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Your molecule is sodium cyanide an ionic compound.
In the lewis structure of H 2 O there are two single bonds around oxygen atom. What is the formal charge on the N. If it is the latter sodium then its because this is an ionic compound and we build the lewis structures of the cation and anion separately.
Best Answer Copy The central atom in a Lewis structure is usually the least electronegative atom. In drawing a Lewis structure the central atom is the a. Find the central atom in the e lectron d ot s tructure Lewis Structure using the method you find best.
Lewis structure of water molecule contains two single bonds around oxygen atom. CO 2 Total 16. See answer 1 Best Answer.
Draw the lewis structure of cs₂ and then determine the number of nonbonding electron pairs on the central atom. Draw a skeletal structure. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms.
Place least electronegative element in center and draw single bonds from the central atom to other atoms. Yes ОО no Incorrect What is the strongest intermolecular force that would act between HNO molecules. Lewis structure is also known as electron dot structure or lewis dot structure because the valence electrons are represented as dots in the lewis structure of the molecule.
If an atom occurs only once this atom is. There is no lone pair present on the central atom but 3 lone pairs present on each outer atom in the lewis dot structure of becl2. The PH3 Lewis structure has 8 valence electrons.
Dipole-dipole interactions hydrogen bonding London dispersion forces Incorrect. Chemistry questions and answers. H 2 O lewis structure.
Atom with the fewest electrons b. Furthermore What is the formal charge. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
A-1 B 0 C 1 D 2 E-2 23 Draw the best Lewis structure for the free radical NO2. Determine the total number of valence electrons to be depicted in the Lewis diagram. Atoms and Atomic Structure.
The formal charge on central Cl atom is -1. What is the role of the central atom when drawing the Lewis structure for. Remember that hydrogen H only needs two valence electrons to have a full outershell.
What is the formal charge on the central Cl atom. First and foremost carbon EN25 is less electronegative than nitrogen EN31 so by your rule it should be in the center. The Lewis structure for PH3 is similar the the structure for NH3 since both P and N.
The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. Write the Lewis structure for CH2O where carbon is the central atom. Chemistry questions and answers.
Atom with the greatest mass c. 22 Draw the best Lewis structure for Cl3. Determine which atom will be the.
Atom with the highest atomic number d. To know the valence electrons of hcn let us go through the valence electrons of individual atoms in hydrogen cyanide. If all of the atoms usually form the same number of bonds the least electronegative atom is usually the central atom.
It is also the atom having low electronegativity. Metals will almost always be the central atom if they are present. Choose a central atom well start with small molecule examples for which there is only one central atom and the other atoms - the peripheral atoms -.
The central atom in a Lewis structure is usually the least electronegative atom.

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
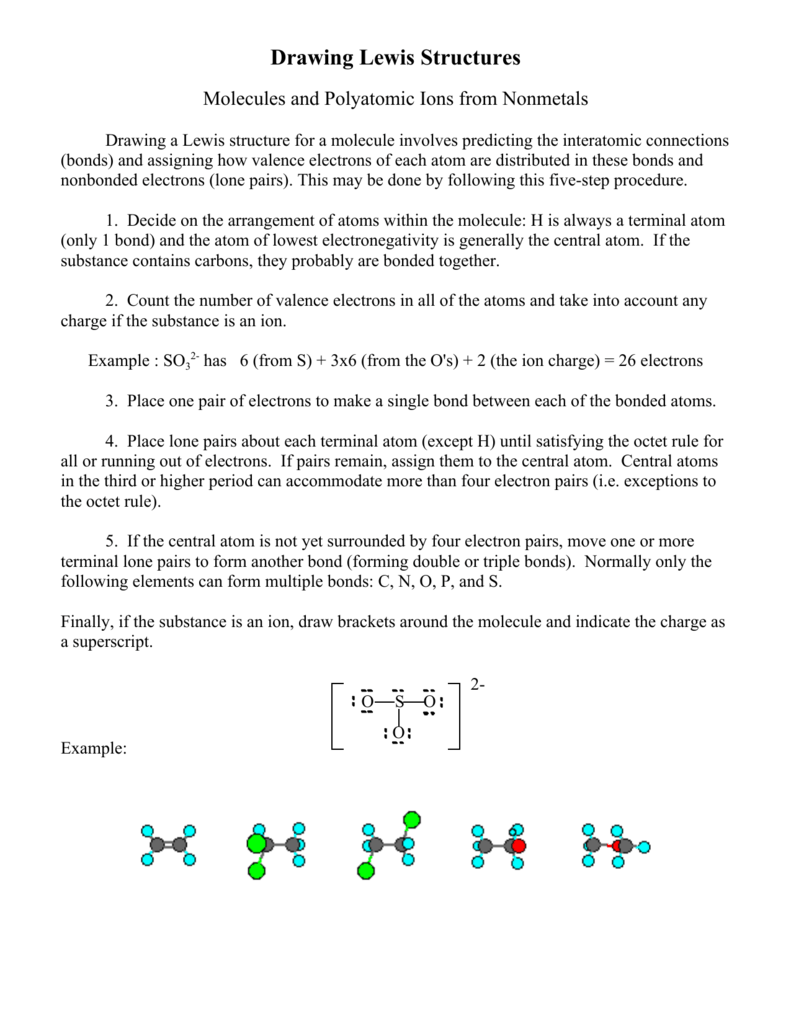
Rules For Drawing Lewis Structures

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

Lewis Structures Chapter 8 Lewis Structures Lewis Structures Are Representations Of Molecules Showing All Valence Electrons Bonding And Nonbonding Ppt Download
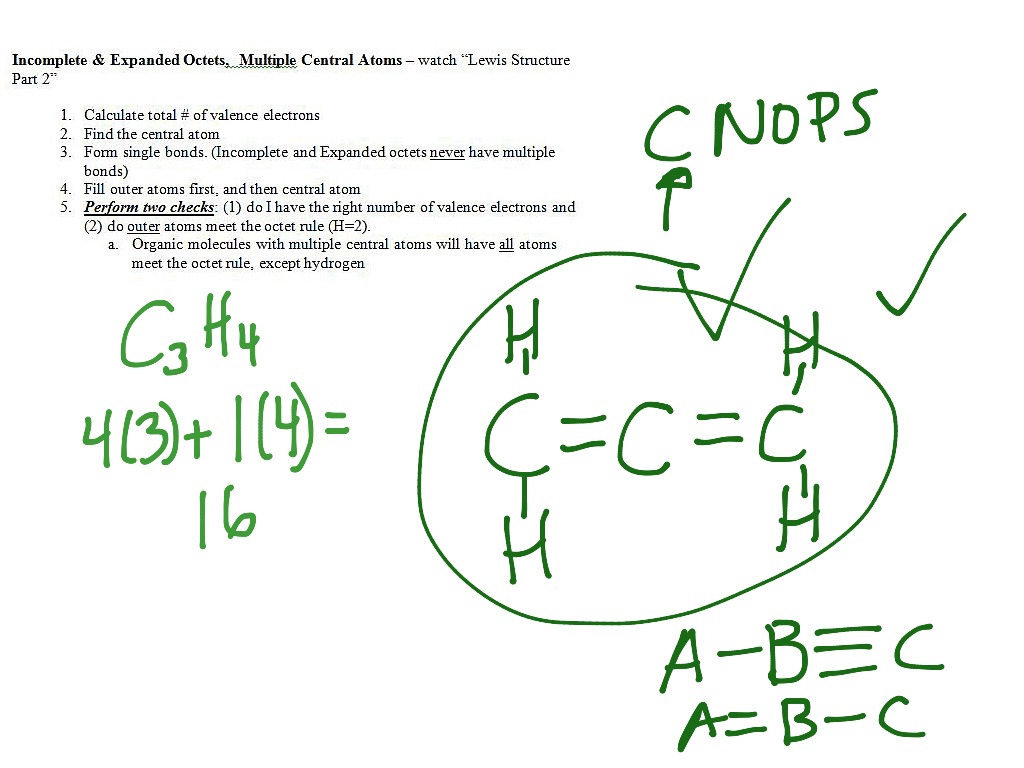
Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

Rules For Lewis Structures Pre Ap Adapted From Brown And Lemay Ppt Download
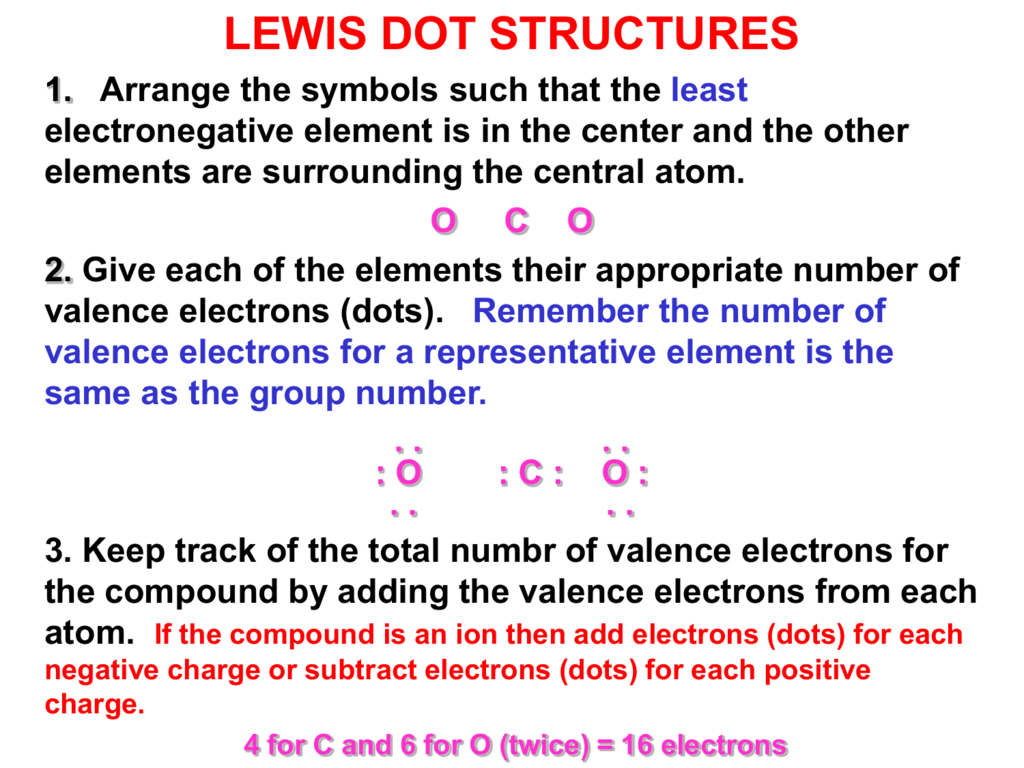

0 komentar
Posting Komentar